PV-related symptoms are prevalent and may impact your patient’s quality of life1
Fatigue
Concentration problems
Early satiety
Itching
Inactivity
Night sweats
Abdominal discomfort
Bone pain
Weight loss
Fever
Based on survey results derived from the Myeloproliferative Neoplasm Symptom Assessment Form assessing 1425 patients with MPNs.1
PV symptoms may impact patients and can exist despite treatment2-4
In the MPN Landmark Survey,*
of patients with PV reported that their symptoms reduced their quality of life2†
*The MPN Landmark Survey, funded by Incyte, was a web-based questionnaire composed of 65 multiple-choice questions intended to help evaluate the patient disease burden in the MPN disease setting. A total of 813 patients in the United States with a previous diagnosis of MF, PV, or ET completed the survey (MF, n=207; PV, n=380; ET, n=226).2
†Patients reported whether they strongly agreed, somewhat agreed, somewhat disagreed, or strongly disagreed with the following statement: PV symptoms reduce my quality of life.2

In [a] study of over 1300 patients that looked at hydroxyurea use in patients, we saw that the majority of patients, despite treatment with hydroxyurea, were still having symptoms.
Lindsey Lyle, MS, PA-C

I cannot emphasize enough that PV-related symptoms are prevalent and [may] impact your patient’s quality of life. Based on my own experience, if you ask specific, detailed questions about PV-related symptoms—such as fatigue, night sweats, or pruritus, among others—you may find that your patients are experiencing one or more [of] these symptoms to some degree.
Prithviraj Bose, MD, MPN Expert White Paper—Optimizing the Management of Patients with Polycythemia Vera
On average, patients with known HU use had moderately high symptom burden (TSS=29.2)3
- A prospective study of 1334 patients with PV in which a subset of patients received HU (n=499)3‡
MPN-10 Mean Symptom Scores in Patients With Known HU Use3
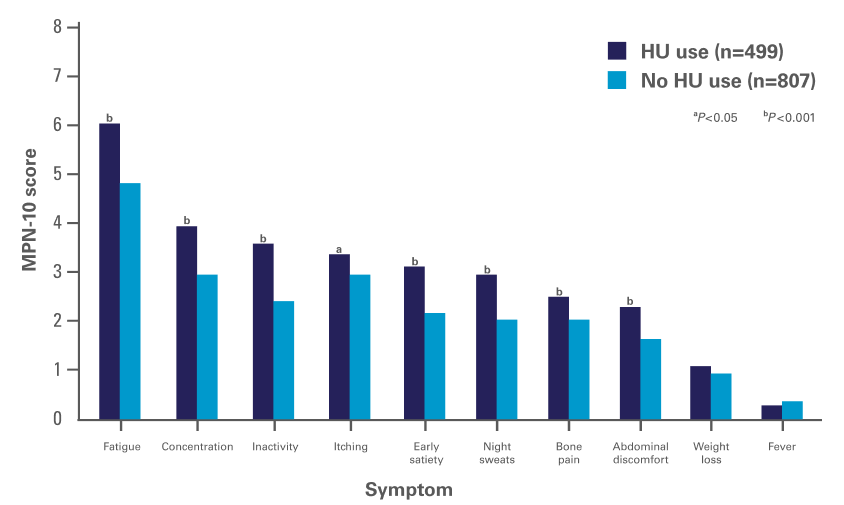
Adapted with permission from Geyer H, Scherber R, Kosiorek H, et al, Symptomatic profiles of patients with polycythemia vera: implications of inadequately controlled disease, Journal of Clinical Oncology, 34(2), pages 151-159. https://ascopubs.org/journal/jco. © 2015 American Society of Clinical Oncology.
‡ A prospective study of 1334 patients with PV was conducted to assess baseline symptoms with certain disease features—known HU use (n=499), known PBT (n=646), palpable splenomegaly (n=369), or all 3 features (n=148)—and compare them to symptoms in a control group of patients who lacked the specified feature. Assessment of MPN symptoms was performed by using the MPN-Symptom Assessment Form Total Symptom Score (MPN-SAF TSS). All items were evaluated on a 0 (absent) to 10 (worst imaginable) scale. The MPN-SAF TSS has a possible range of 0 to 100, with 100 representing the highest level of symptom severity. The TSS for each patient was analyzed to place the patient into the quartiles of low symptom burden (TSS, 0 to 7), intermediate symptom burden (TSS, 8 to 17), moderately high symptom burden (TSS, 18 to 31), or high symptom burden (TSS, ≥32).3

Hear from Robyn Scherber, MD, MPH, about the importance of looking beyond blood counts and actively monitoring PV symptoms
Patients with PV had moderately high symptom burden regardless of blood count control4
- Symptom burden in patients who achieved blood count control vs those who did not was analyzed among 1813 evaluable patients with PV in the prospective, observational REVEAL study4§
Mean TSS According to Blood Count Control Status (Hct, WBC, PLT)4
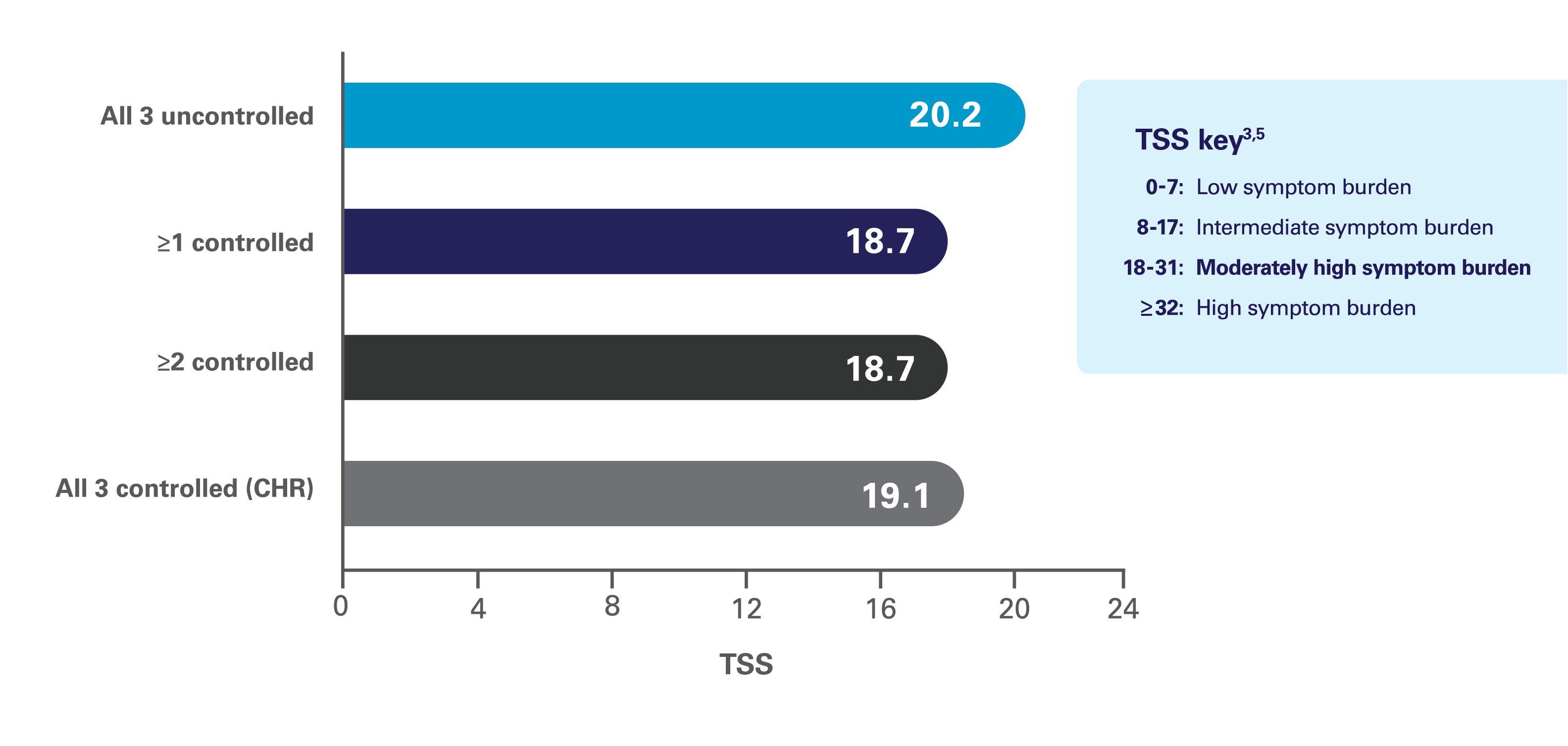
Reprinted from Clinical Lymphoma, Myeloma & Leukemia, 19(9), Grunwald MR, Burke JM, Kuter DJ, et al, Symptom burden and blood counts in patients with polycythemia vera in the United States: an analysis from the REVEAL study, Pages 579-584, © 2019, with permission from Elsevier.

In polycythemia vera, blood counts and symptoms can be independent factors. Regardless of what a patient’s blood counts are, or whether or not their disease is well controlled, they can still have severe symptom burden.
Robyn Scherber, MD, MPH
§ REVEAL was a prospective, observational study that collected contemporary data regarding burden of disease, clinical management, patient-reported outcomes, and healthcare resource utilization from adult patients with PV in the United States, and it was sponsored by Incyte. A total of 2510 patients were enrolled over an approximate 2-year period (July 2014 to August 2016), with 2307 patients having completed the MPN-SAF TSS at enrollment. Of these, 1813 (72.2%) had a complete blood count within 30 days before completion of the at-enrollment MPN-SAF TSS and were evaluable. At the time of enrollment, most patients (n=1714; 94.5%) were being managed with cytoreductive therapy; 1581 patients (87.2%) were managed with PBT, HU, or a combination thereof. CHR was defined as Hct <45%, WBC count <10 × 109/L, and PLT count ≤400 × 109/L; these same criteria were used to determine if Hct, WBC, and PLT were controlled.4
CHR=complete hematologic remission; ET=essential thrombocythemia; Hct=hematocrit; HU=hydroxyurea; MF=myelofibrosis; MPN=myeloproliferative neoplasm; PBT=phlebotomy; PLT=platelet; PV=polycythemia vera; REVEAL=pRospective obsErvational study of patients with polycythemia VEra in US clinicAL practices; TSS=Total Symptom Score; WBC=white blood cell.


