Hct ≥45% and elevated WBC counts are key clinical considerations for thrombotic risk that may be overlooked1,2
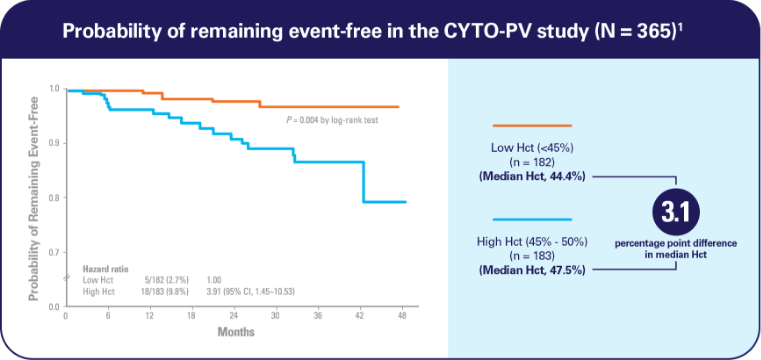
Hct levels ≥45% carry a serious risk in PV1
Elevated Hct between 45% and 50%: 4-fold higher rate of cardiovascular death and major thrombosis1
- Managing Hct levels between 45% and 50% significantly increased the risk of cardiovascular death and major thrombosis compared with an Hct level managed to <45% (HR, 3.91; 95% CI, 1.45-10.53; P = 0.007)1*
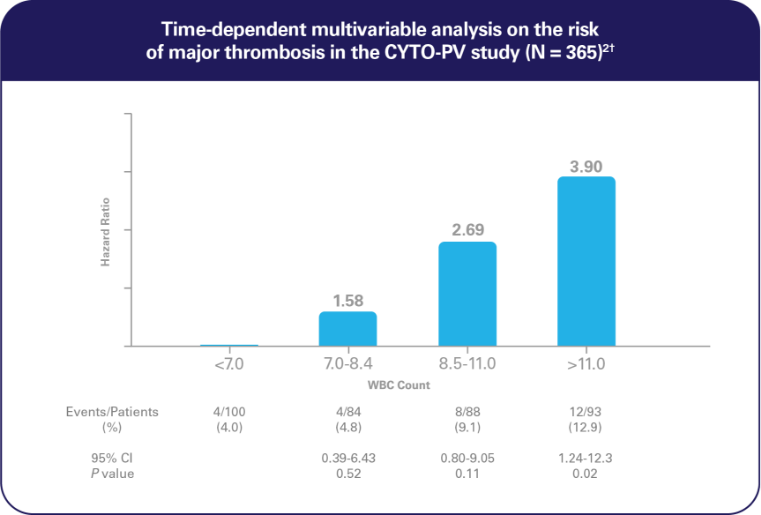
WBC counts are an independent risk factor for thrombosis2
In an additional analysis from the CYTO-PV study,
Elevated WBC counts >11 × 109/L increased the risk of thrombosis2
- In a multivariable, time-dependent analysis, WBC counts >11 × 109/L were associated with increased risk of thrombosis (HR, 3.9; 95% CI, 1.24-12.3; P = 0.02)2

In our practice, elevated WBCs and/or progressive leukocytosis serve as indications for the initiation of cytoreductive therapy or a change in cytoreductive therapy to effectively manage the patient’s thrombotic risk.
—Srdan Verstovsek, MD, PhD
White Paper—Leukocytosis and the Threat of Thrombosis in PV
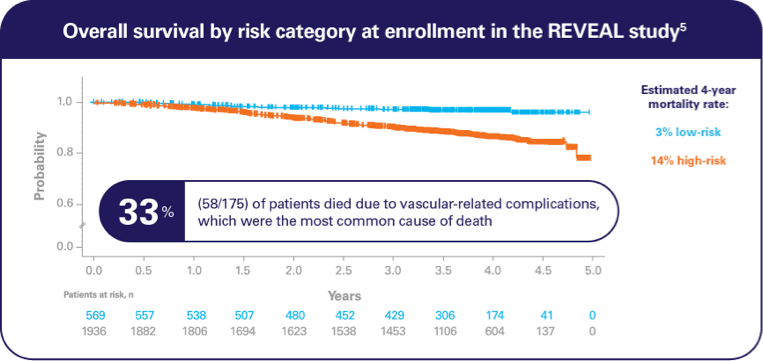
In the prospective, observational, REVEAL‡ study,
The estimated 4-year mortality rate was 14% in patients with high-risk PV5§
- 86% of high-risk patients (1660/1940) received HU and/or PBT at enrollment6
Reprinted from Chest, 156(2), Benza RL, Gomberg-Maitland M, Elliott CG, et al, Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies, 323-337, Copyright 2019, with permission from Elsevier.
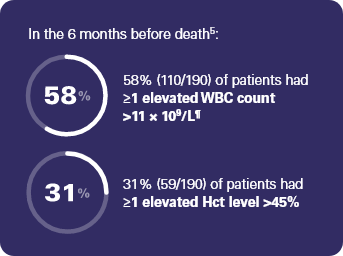
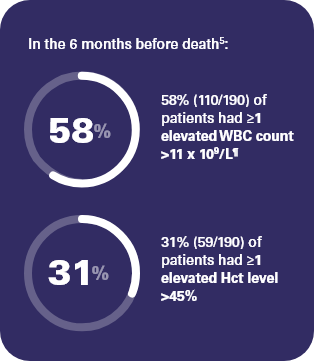
‡ REVEAL was a prospective, observational study of 2510 adult patients with PV in the US, sponsored by Incyte. Patients were enrolled over an approximate 2-year period (July 2014 to August 2016). The analysis included all enrolled patients and evaluated characteristics of deceased patients, survival by risk, and causes of death over the course of the study. A total of 244 patients died during the study, with 190 having available Hct values and WBC counts in the 6 months before death, and 175 having a known cause of death. Among the 244 patients who died during the study, 82% (n = 200) were categorized as high-risk at diagnosis, primarily due to age ≥60 years only (65%; n = 159).5
§ 77% of patients (1940/2510) were classified as high-risk at enrollment based on age ≥60 years and/or history of thrombotic events.6
¶ 71% (78/110) of these patients did not experience an infection in the year prior to death.7
In the REVEAL study,
Some patients continued to have elevated blood counts, despite treatment with HU alone and HU plus PBT8,9
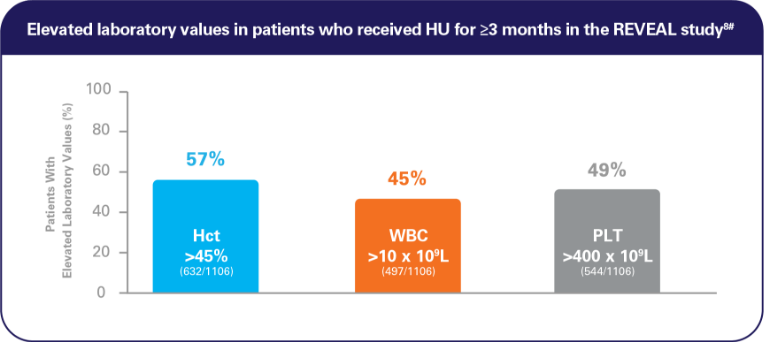
Analysis included patients with all 3 laboratory values.8
Reprinted from Clinical Lymphoma Myeloma and Leukemia, 20(4), Grunwald MR, Kuter DJ, Altomare I, et al, Treatment Patterns and Blood Counts in Patients with Polycythemia Vera Treated With Hydroxyurea in the United States: An Analysis From the REVEAL Study, 219-225, Copyright 2020, with permission from Elsevier.
PLT, platelet.
# REVEAL was a prospective, observational study of 2510 patients with PV in the US, sponsored by Incyte. This analysis focused on blood count control in the subset of 1381 patients who had received HU for ≥3 months.8
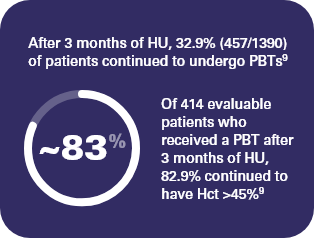
Patients who were treated with HU and receiving PBT were more likely to have elevated blood counts9
The median of the maximum Hct value among evaluable patients (n = 1106) who received HU for ≥3 months was 48% for those who reported a value of >45% and 42% for those who reported a value ≤45%9
References: 1. Marchioli R, Finazzi G, Specchia G, et al; CYTO-PV Collaborative Group. N Engl J Med. 2013;368(1):22-33. 2. Barbui T, Masciulli A, Marfisi MR, et al. Blood. 2015;126(4):560-561. 3. Gangat N, Strand J, Li CY, Wu W, Pardanani A, Tefferi A. Br J Haematol. 2007;138(3):354-358. 4. Landolfi R, Di Gennaro L, Barbui T, et al; for the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Blood. 2007;109(6):2446-2452. 5. Stein BL, Patel K, Scherber RM, Yu J, Paranagama D, Miller CB. Presented at: 62nd ASH Annual Meeting and Exposition; December 5-8, 2020. Abstract 484. 6. Grunwald MR, Stein BL, Boccia RV, et al. Clin Lymphoma Myeloma Leuk. 2018;18(12):788-795. 7. Data on file. Incyte Corporation. Wilmington, DE. 8. Grunwald MR, Kuter DJ, Altomare I, et al. Clin Lymphoma Myeloma Leuk. 2020;20(4):219-225. 9. Grunwald MR, Altomare I, Burke JM, et al. Poster presented at: 59th ASH Annual Meeting and Exposition; December 9-12, 2017; Atlanta, GA. Poster 1633.



